Validation Lifecycle Management Systems (VLMS) Implementation
Life Sciences Organizations engaged in drug development face an increasingly challenging environment: an expanding global footprint for clinical trials, evolving regulatory requirements, dynamic partnership models, and rapidly evolving technology. At the same time, many organizations are also innovating how trials are designed and conducted, adding to the complexity. Managing this complexity could be greatly enhanced by an effective and efficient Validation Life Cycle Management Solution (VLMS) and Quality Management System (QMS). However, what constitutes an effective and efficient QMS is not well defined for clinical development. Cereblis QMS Consulting Services helps to develop a flexible, holistic, clinical QMS framework to address this gap and helps Quality Management System Design, Gap Analysis, Implementation, and Improvement.
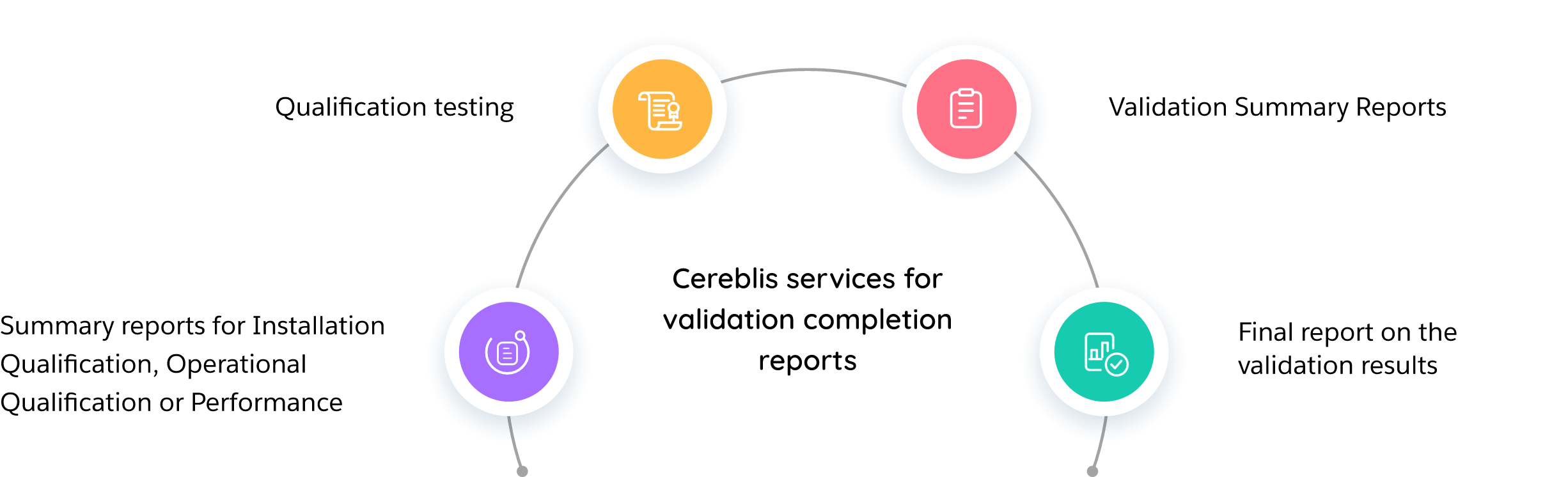
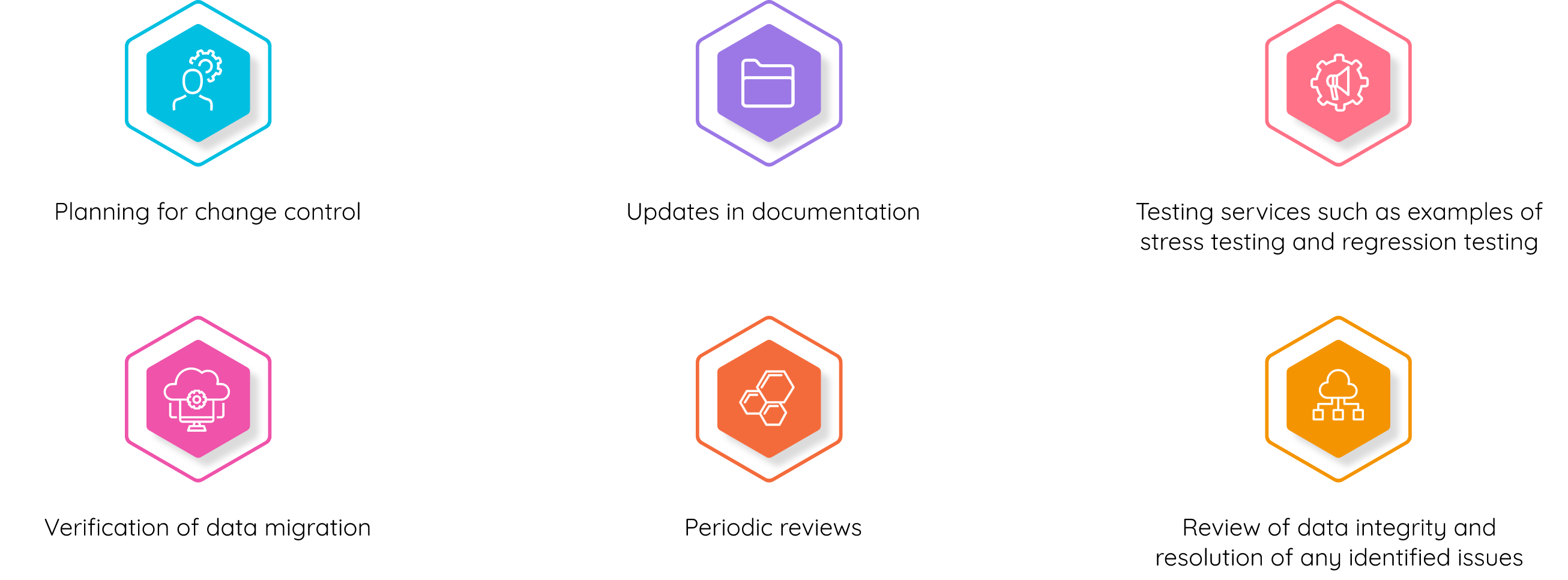
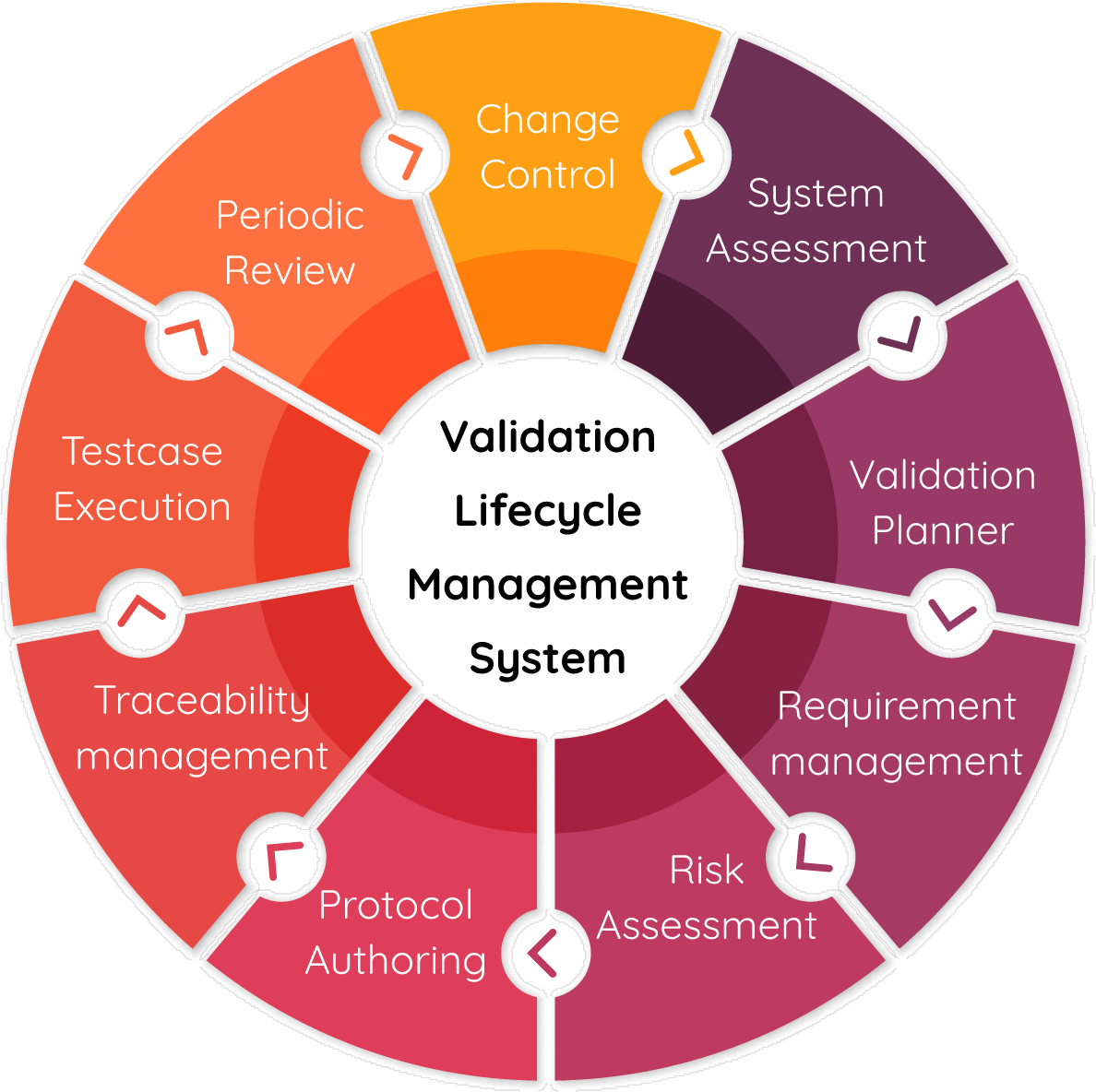
Title 21 CFR Part 11 requires companies to implement controls, including audits, system validations, audit trails, electronic signatures, and documentation for software and systems that are involved in processing many forms of data, as part of their business practices and product development. We will help you to adopt a digital system of record-keeping while being compliant & complete the digital transformation of your QMS. Cereblis will help you identify, advise and implement innovative Validation Life Cycle management (VLMS) solution to address your 21 CFR Part 11, 21 CFR part Annex 11, GAMP 5, Change management, Release Management, Risk Assessment and digitize IQ, OQ, and PQ documentation requirements.

Life Sciences Organizations engaged in drug development face an increasingly challenging environment: an expanding global footprint for clinical trials, evolving regulatory requirements, dynamic partnership models, and rapidly evolving technology. At the same time, many organizations are also innovating how trials are designed and conducted, adding to the complexity. Managing this complexity could be greatly enhanced by an effective and efficient Computer System Validation (CSV) and Quality Management System (QMS).
Computer systems validation (CSV) is a critical requirement from the regulatory perspective (U.S. FDA, EMA e.t.c). The validation process is designed to provide a high degree of assurance that both new and existing computer systems will consistently fulfill their intended purpose by producing results which meet predetermined specifications and quality attributes – accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records.
As Life Sciences applications constantly evolve to keep up with the needs of the people and businesses that use them, Life Science companies must perform validation activities on an ongoing basis in order to reduce compliance risk, ensure quality, and maintain data integrity.A “Computer System” in an FDA regulated laboratory is more than just computer hardware and software – it also includes any equipment and instruments linked to the system, as well as the trained staff that operate the system and/or equipment using Standard Operating Procedures (SOPs) and manuals. Computer system validation requires a comprehensive set of both static and dynamic testing activities that must be conducted throughout the Software Document Life Cycle (SDLC)
At Cereblis, we are experts in IT risk identification and management along with regulatory compliance. As such, we understand that computer system validation is not a “one size fits all” process. With over 20 years of validation experience, we work to create CSV processes that are based on the latest FDA regulations and guidance (GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems), best practices, and the characteristics of the system being validated.

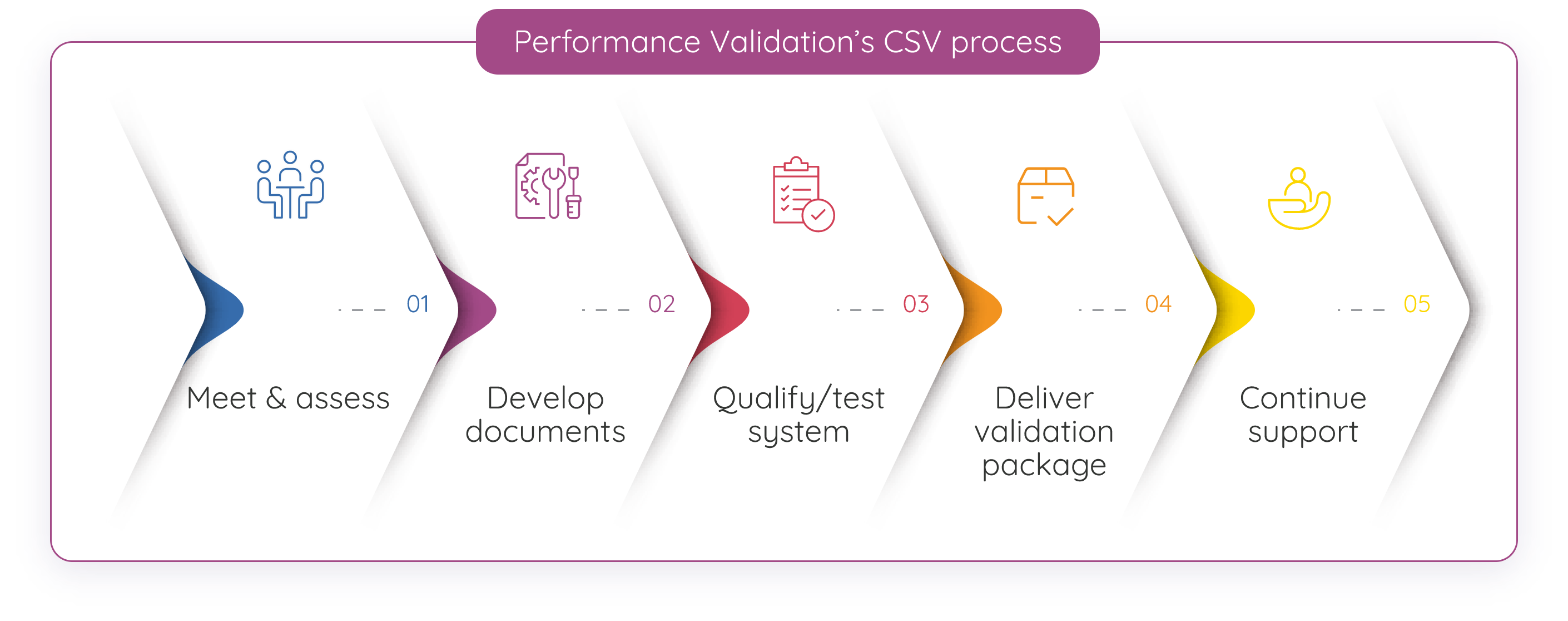
Our CSV process
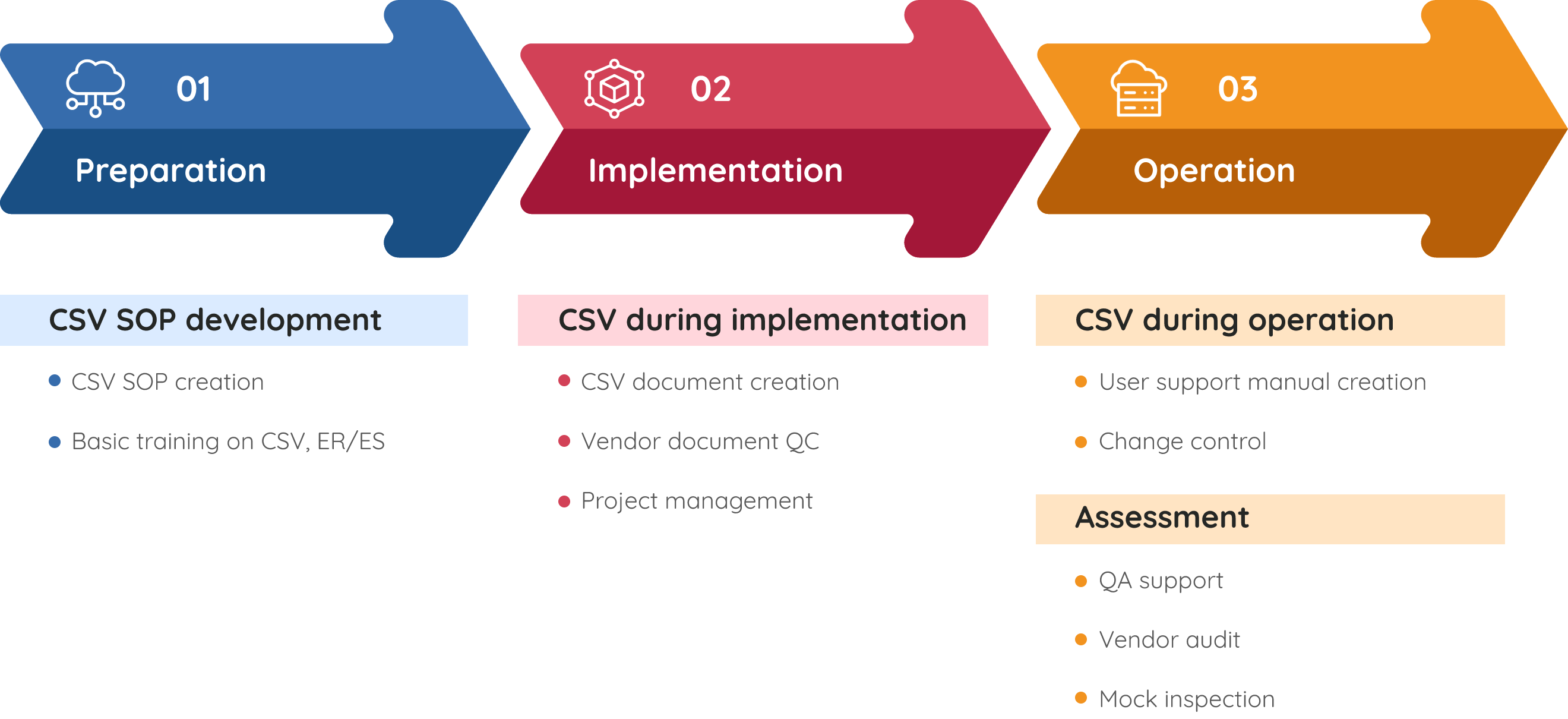
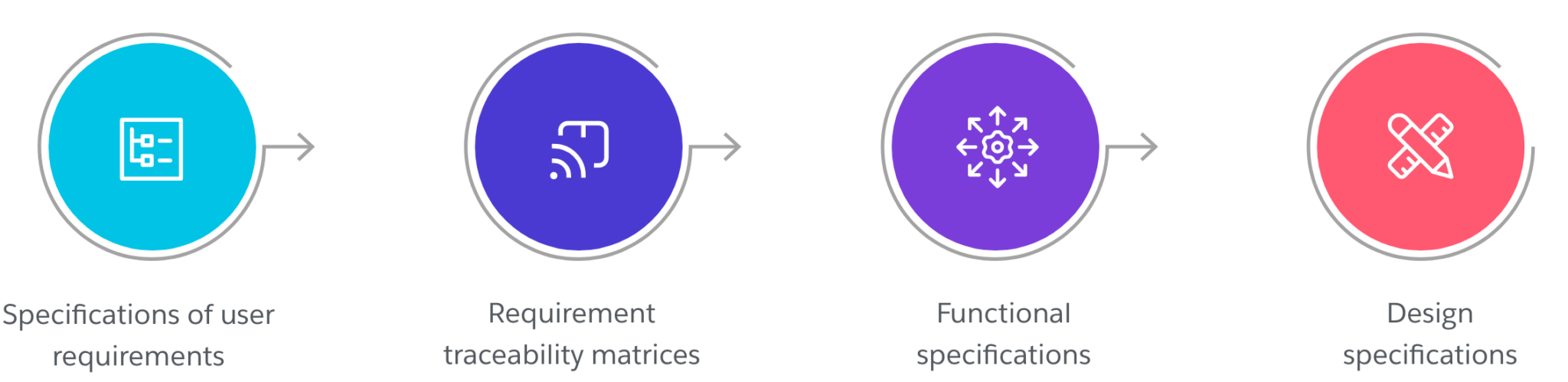
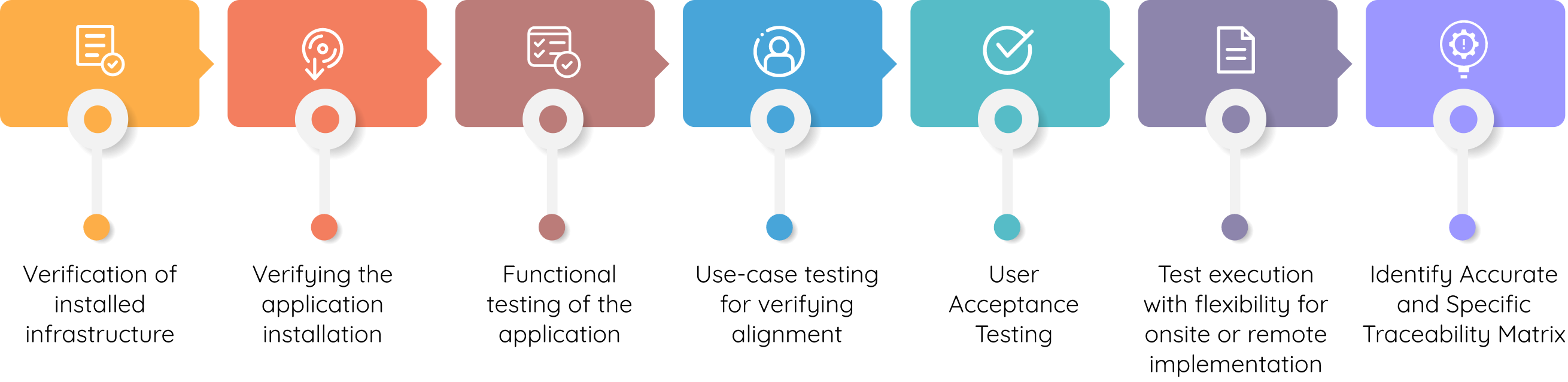
Our Validation Approach