
In the realm of clinical research, Investigator Initiated Trials (IIT) and Investigator Initiated Research (IIR) play a pivotal role in advancing medical knowledge and improving patient care. These initiatives empower investigators and research institutions to take the lead in designing and conducting clinical trials and studies. To streamline the management of IIT, IIR, and the associated grants, many organizations are turning to technology solutions like Salesforce Experience Cloud. In this blog post, we’ll explore what IIT, IIR, and Grants Management entail and how Salesforce Experience Cloud can facilitate their digitization.
Investigator Initiated Trials (IIT) and Investigator Initiated Research (IIR)
Investigator Initiated Trials (IIT) refer to clinical trials where the investigator (usually a physician or researcher) takes the lead in designing, conducting, and often funding the trial. These trials are initiated by investigators rather than pharmaceutical or medical device companies. IITs are valuable because they enable researchers to explore novel treatment options, test hypotheses, and address critical clinical questions that may not align with the priorities of industry-sponsored trials.
Investigator Initiated Research (IIR) is a broader term that encompasses research projects beyond clinical trials. It includes observational studies, epidemiological investigations, and basic science research. Like IITs, IIR allows investigators to pursue their research interests independently.
Both IIT and IIR hold immense potential for advancing medical science, but managing these initiatives and the associated grants can be complex and challenging without the right tools in place.
Grants Management in IIT and IIR
Effective Grants Management is crucial for the success of IIT and IIR programs. Research institutions and sponsors need to track and allocate funds, manage budgets, monitor progress, and ensure compliance with regulatory requirements and ethical standards. Here are some key aspects of Grants Management in IIT and IIR:
- Proposal Submission: Investigators submit research proposals, including budgets and project plans, to funding organizations or internal review committees.
- Review and Approval: Proposals are reviewed and evaluated based on scientific merit, feasibility, and alignment with the organization’s goals. Approved projects receive funding.
- Budgeting and Finance: Managing grant budgets, disbursing funds, tracking expenses, and ensuring financial compliance are critical components of Grants Management.
- Project Monitoring: Ongoing monitoring of research progress, adherence to timelines, and compliance with regulations is essential to ensure the success and integrity of the research.
- Reporting and Documentation: Comprehensive documentation, including progress reports, financial statements, and adherence to ethical guidelines, is necessary for accountability and reporting to sponsors and regulatory bodies.
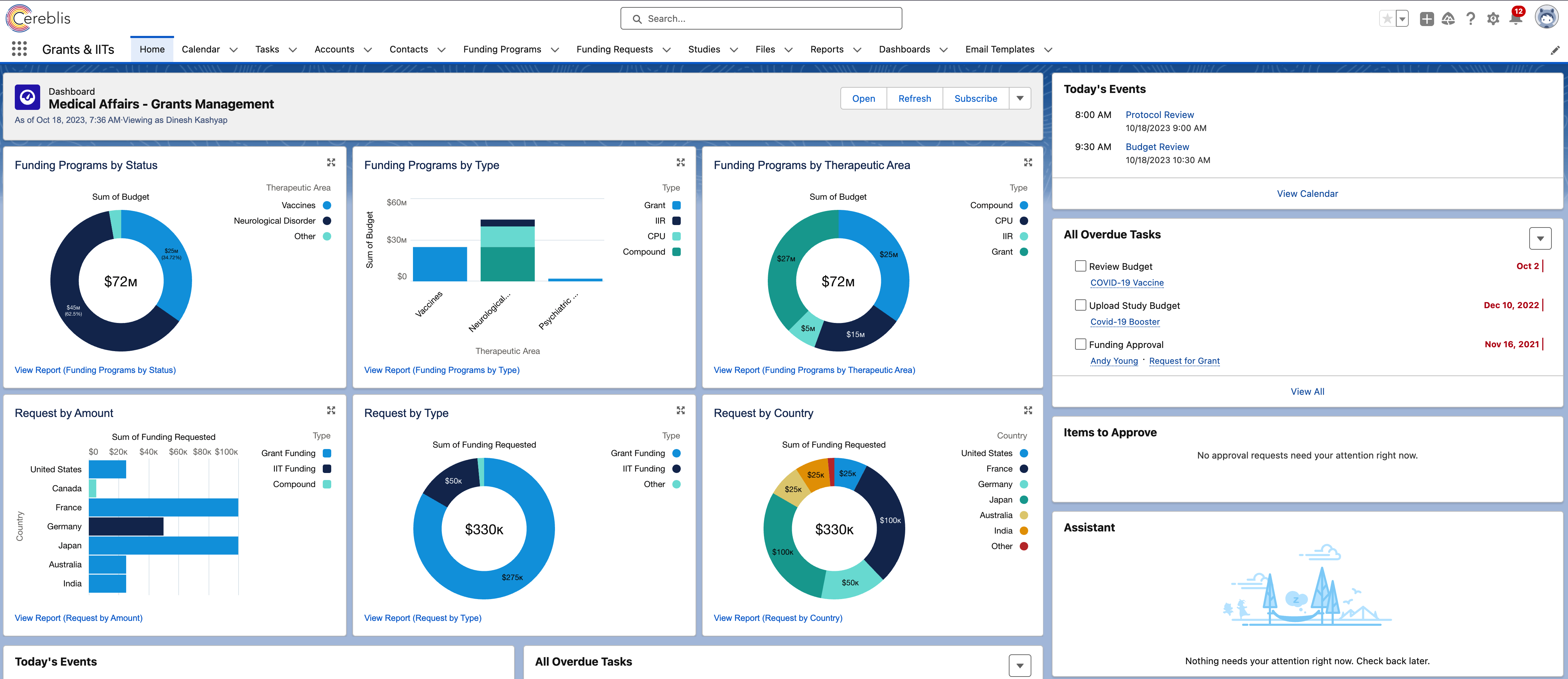
Digitizing IIT, IIR, and Grants Management with Salesforce Experience Cloud
Salesforce Experience Cloud offers a powerful solution for digitizing the management of IIT, IIR, and Grants. Here’s how Salesforce Experience Cloud can be leveraged:
1. Centralized Data Repository
Salesforce Experience Cloud provides a centralized repository for all IIT, IIR, and Grants Management data. This includes investigator profiles, proposal submissions, funding information, budgets, and compliance documents. Having all this information in one place enhances visibility and accessibility for stakeholders.
2. Streamlined Proposal and Approval Workflows
Salesforce Experience Cloud enables the creation of custom workflows for proposal submission, review, and approval. Investigators can submit proposals online, and review committees can collaborate and make decisions within the platform. Automated notifications and reminders help keep the process on track.
3. Budgeting and Financial Management
Salesforce Experience Cloud’s financial tools allow for the management of grant budgets, expense tracking, and financial reporting. Real-time data updates ensure that budgets are effectively managed, and potential issues are identified early.
4. Collaboration and Communication
The platform facilitates collaboration among investigators, sponsors, and research teams. Secure communication channels and document sharing capabilities ensure that all stakeholders are on the same page throughout the research process.
5. Compliance Tracking
Salesforce Experience Cloud allows for the creation of custom compliance checklists and tracking mechanisms. This ensures that all research activities adhere to regulatory and ethical standards, reducing the risk of compliance issues.
6. Reporting and Analytics
Robust reporting and analytics tools in Salesforce Experience Cloud provide insights into the progress of IIT and IIR projects. Customizable dashboards and reports enable stakeholders to monitor key performance indicators and make data-driven decisions.
7. Mobile Accessibility
With mobile-friendly features, Salesforce Experience Cloud allows users to access critical information and perform tasks on the go, making it convenient for investigators and research teams.
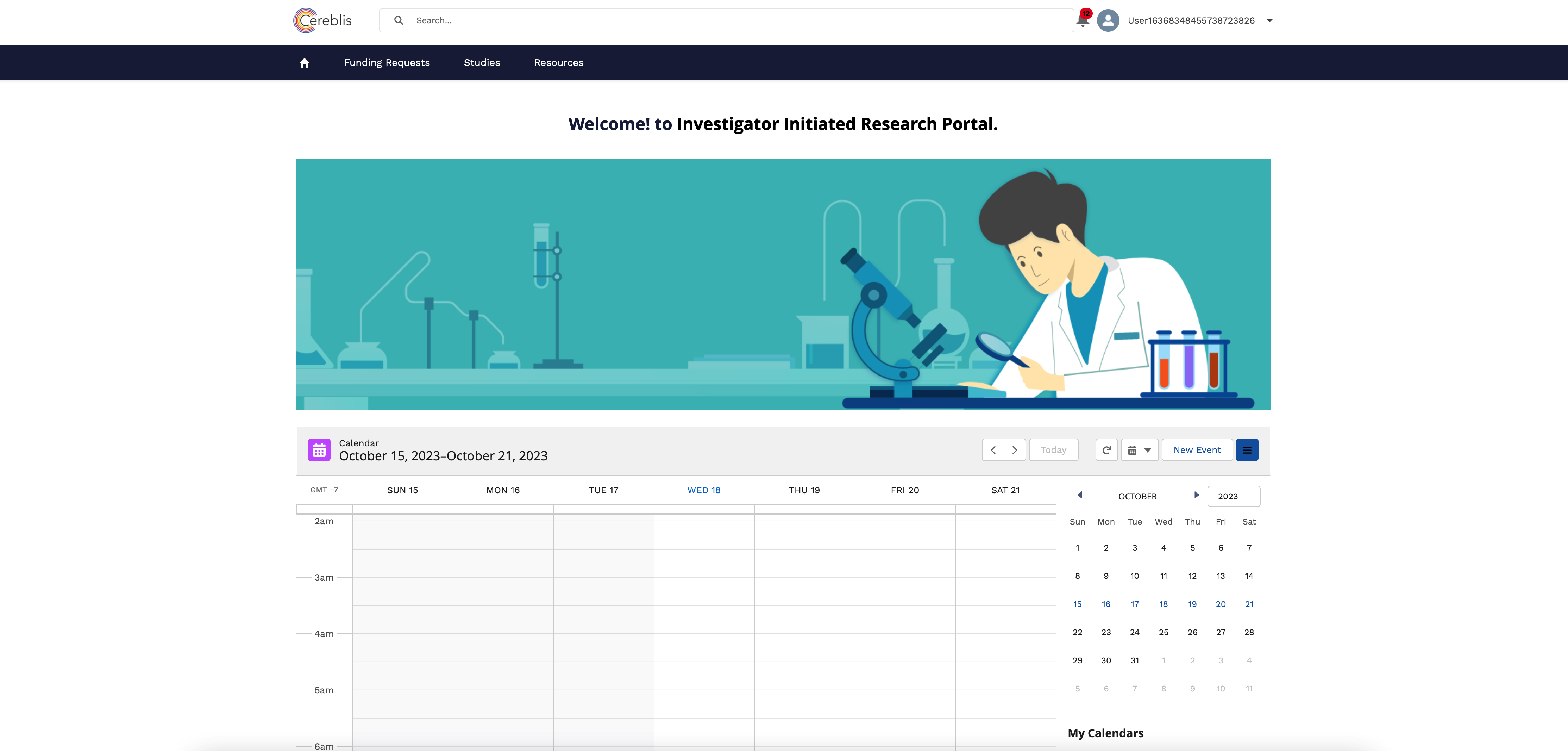
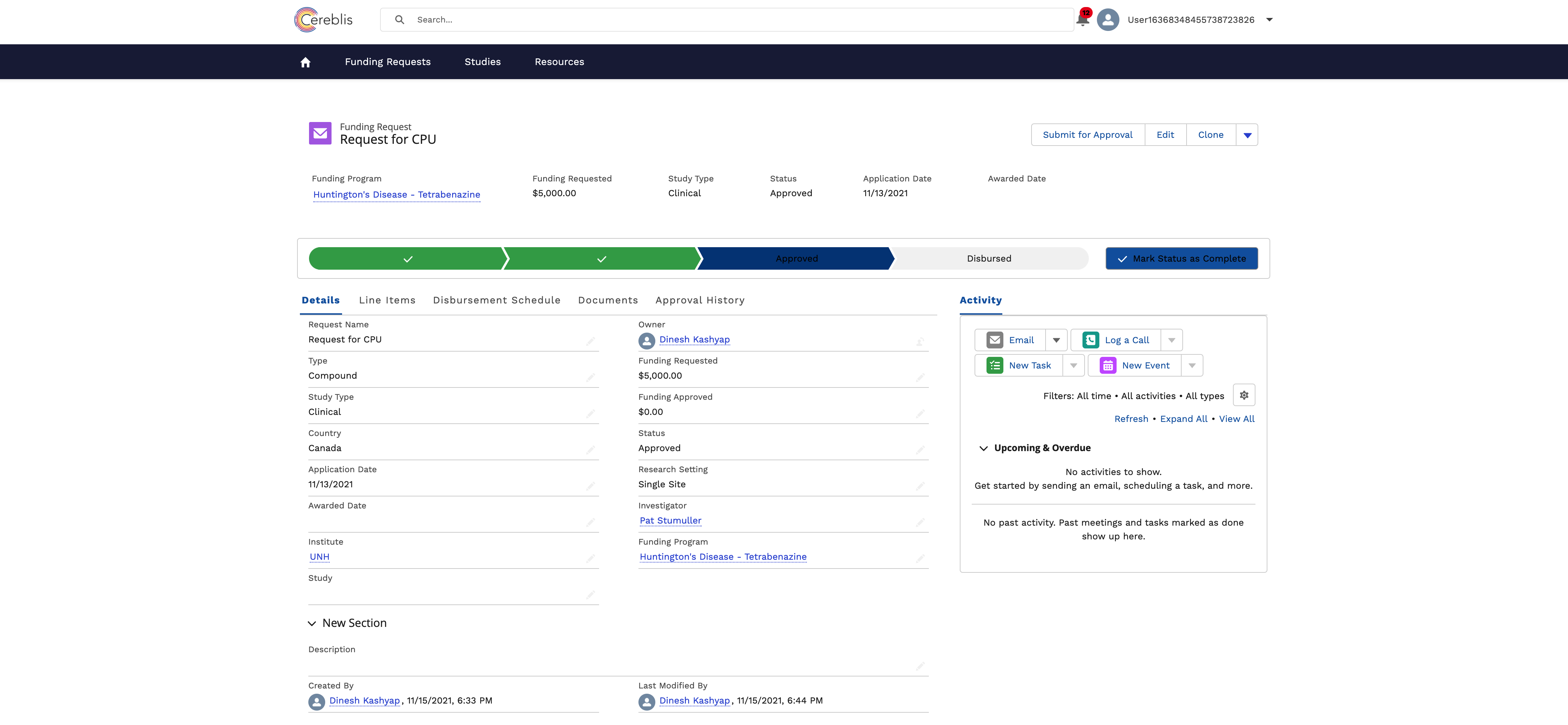
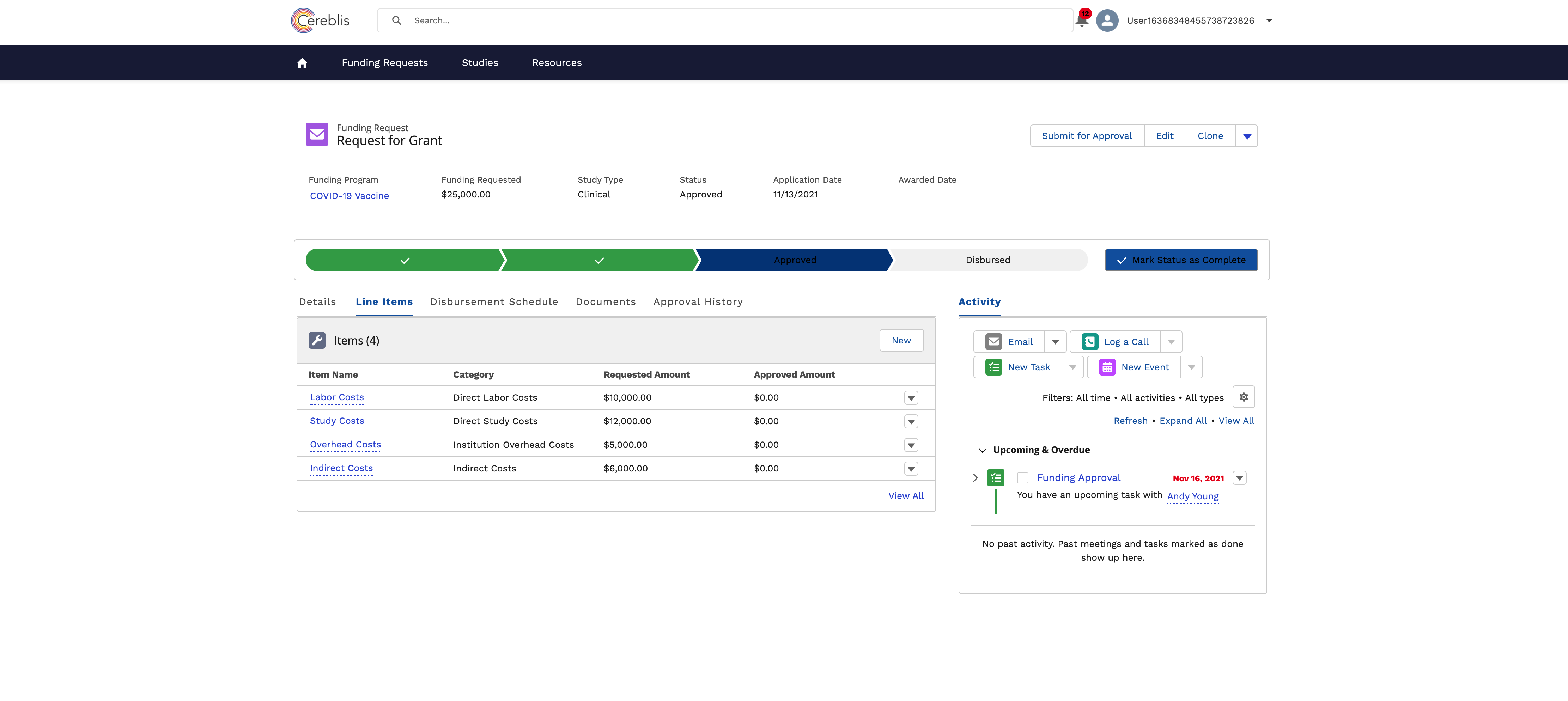
In conclusion, Investigator Initiated Trials (IIT), Investigator Initiated Research (IIR), and Grants Management are vital components of advancing medical research. Salesforce Experience Cloud offers a comprehensive solution for digitizing and streamlining these processes, ensuring efficient management, compliance, and collaboration among stakeholders. By harnessing the power of Salesforce Experience Cloud, research institutions can accelerate the pace of discovery and make a meaningful impact on patient care and scientific knowledge.


